Technology
Working principle
Hydrogen molecules are flowing through the electrochemical cell, where they make contact to the catalyst.
This catalyst allows electrons to be dissociated from the hydrogen molecule, leaving 2 protons (H+).
These protons are absorbed and transported through the membrane to the cathode side, because there electrons are available.
Once arrived at the cathode side, another catalyst material recombines the protons with their respective electrons to form a hydrogen molecule once again.
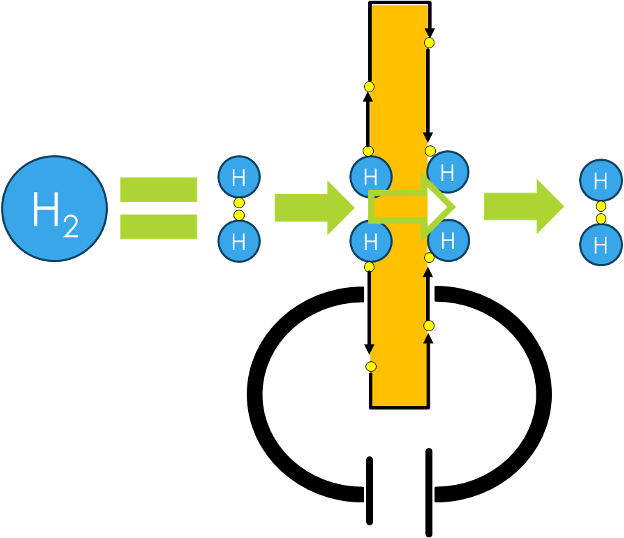
Working principle 2
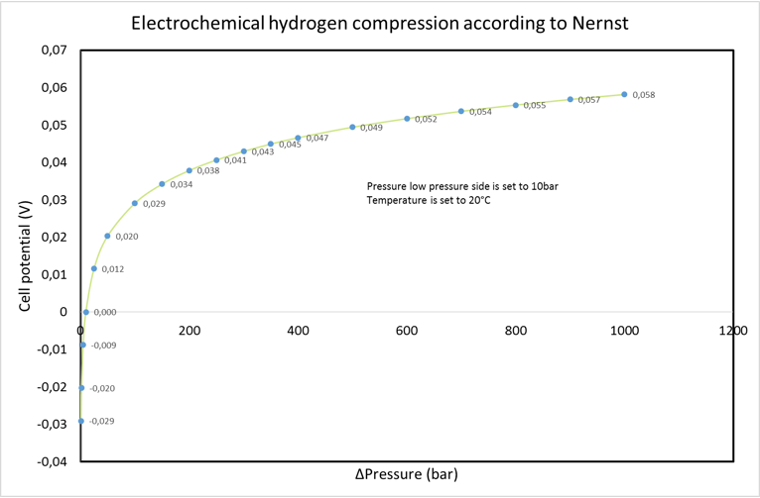
Nernst equation:
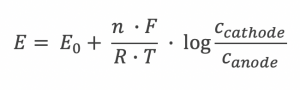
E = Cell potential / Potential difference
E0 = Redox potential
n = number of moles
F = Faradays constant
R = Gas constant
T = Temperature in Kelvin
A typical electrochemical (PEM) cell consists of a proton conducting membrane and a catalyst layer on both sides of the membrane. Also known as a membrane electrode assembly (MEA). Proton Technologies has managed to optimise its MEA design with unique features and components, giving it superior performance over its competitors. Key features of Proton Technologies MEA design are:
- Low gas permeability
- Extreme high proton conductivity
Reducing your capital expenses on equipment and reducing your operation expenses on energy
